Abstract
Background:
Diffuse Large B-cell Lymphoma (DLBCL), the most common lymphoid cancer, is classified by the cell of origin into the germinal and non-germinal center (non-GCB) subtypes. The non-GCB subtype is associated with inferior outcomes with standard therapies. BTK inhibitors like acalabrutinib (A) and the immunomodulatory agent lenalidomide (L) have activity as single agents, and result in synthetic lethality when combined in non-GCB DLBCL models. Both the CD19 antibody tafasitamab (T) and CD20 antibody rituximab (R) demonstrated significant clinical activity with L in patients with relapsed DLBCL. L, T, R, and A are also immunomodulatory, shifting from a tumor-mediated immune anergy to an anti-tumor immune response. In a previous phase II trial (Smart Start) in high risk newly diagnosed non-GCB DLBCL patients, the combination of RL+the BTK inhibitor, ibrutinib (I) demonstrated an overall response rate (ORR) of 86% prior to chemotherapy with a 2 year OS of 96% following RLI + chemotherapy. One patient withdrew consent after 2 cycles of RLI alone and is now >3y in a confirmed durable remission without chemotherapy. Together these data suggest that chemotherapy may be abbreviated or eliminated for some patients.
Our current study builds upon our previous experience harnessing synthetic lethality, an optimized immune response, and a response adapted duration of chemotherapy.
Study Design and Methods:
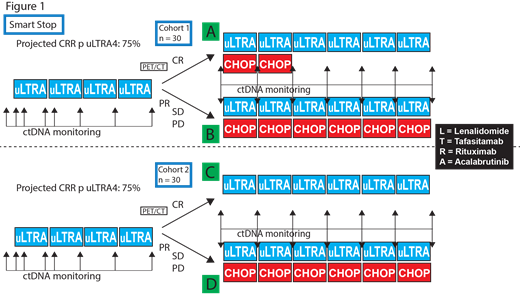
We are conducting a phase II, open-label, single-center clinical trial evaluating LTRA alone and combined with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) for patients with previously untreated non-GCB DLBCL (NCT04978584).
Eligibility criteria include patients who are 18y+ with previously untreated DLBCL intended for full dose therapy, IHC defined non-GCB subtype with adequate organ and bone marrow function. Patients with active viral infections, known CNS involvement with lymphoma, recent thrombosis or inability to tolerate prophylactic anticoagulation are ineligible.
Patients will receive lenalidomide (L) 25mg PO days 1-10, tafasitamab (T) 12mg/kg IV weekly, rituximab (R) 375mg/m2 IV day 1, and acalabrutinib (A) 100mg PO twice daily during a 21 day cycle. All patients will receive the LTRA combination for four cycles, followed by restaging studies. If there is clinical suspicion of progression of disease prior to completing cycle 4, restaging and potential initiation of CHOP may proceed earlier if necessary. Response will be assessed via PET/CT scan using the 5 point Deauville score (5PS) with CR defined as a 5PS of 1, 2, or 3. Secondary response endpoints will include total metabolic tumor volume and total lesion glycolysis in comparison with baseline, and assessment of ctDNA based upon the CAPP-Seq approach.
Patients who achieve a complete response (CR) with 4 cycles of LTRA will receive 6 additional cycles of LTRA (Figure 1, Group A or C). The trial will have two sequential cohorts of 30 patients each. Cohort 1 with CHOP consolidation for 2 cycles (Group A) added to LTRA, and cohort 2 with no CHOP consolidation added to LTRA (Group C) for CR patients. We will utilize two decision rules based upon results from cohort 1 to open and complete cohort 2: 1) the probability in Group A of sustained CRR at the end of therapy, 2) ) the probability in Group A of sustained response at 7 months after end of therapy in the first 10 patients. Patients in either cohort 1 or 2 who achieve less than a CR after 4 cycles of LTRA (Group B or D) will receive LTRA-CHOP for 6 cycles, with a planned total of 10 cycles of therapy for all patients.
The primary objectives are to determine 1A: the ORR after 4 cycles of LTRA and 1B: the CRR at of all LTRA +/-CHOP at the end of therapy. The maximum sample size is 60 patients, with a target CRR for 4 cycles of LTRA of 75%. Secondary objectives include survival outcomes, safety, and outcomes of LTRA without CHOP, LTRA with 2 or 6 cycles of CHOP. Exploratory objectives include determining ctDNA response, immune modulation driven by LTRA, and LTRA response associated characteristics.
Our trial is the first to explore novel-novel combinations with a response adapted number of chemotherapy cycles in newly diagnosed DLBCL patients, potentially identifying patients who may benefit from abbreviated or no chemotherapy.
Westin: Kite, a Gilead Company: Consultancy, Research Funding; Curis: Research Funding; Novartis: Consultancy, Research Funding; Umoja: Consultancy; Morphosys: Research Funding; MorphoSys: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Iksuda Therapeutics: Consultancy; 47 Inc: Research Funding. Steiner: BMS: Research Funding; Seattle Genetics: Research Funding; Rafael Pharmaceuticals: Research Funding. Strati: Roche-Genentech: Consultancy; Astrazeneca-Acerta: Research Funding. Flowers: Takeda: Research Funding; Pharmacyclics/Janssen: Consultancy; SeaGen: Consultancy; Epizyme, Inc.: Consultancy; Xencor: Research Funding; Spectrum: Consultancy; TG Therapeutics: Research Funding; AbbVie: Consultancy, Research Funding; Pfizer: Research Funding; Amgen: Research Funding; Sanofi: Research Funding; National Cancer Institute: Research Funding; Adaptimmune: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Denovo: Consultancy; Nektar: Research Funding; Gilead: Consultancy, Research Funding; Biopharma: Consultancy; Karyopharm: Consultancy; Ziopharm: Research Funding; Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Guardant: Research Funding; BeiGene: Consultancy; Allogene: Research Funding; Genentech/Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Cellectis: Research Funding; Burroughs Wellcome Fund: Research Funding; Janssen: Research Funding; Acerta: Research Funding; Iovance: Research Funding; EMD: Research Funding; Bayer: Consultancy, Research Funding; Novartis: Research Funding; 4D: Research Funding; Morphosys: Research Funding; Kite: Research Funding; Genmab: Consultancy; Pharmacyclics: Research Funding. Neelapu: Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr, Unum Therapeutics and Bluebird Bio: Honoraria; Kite, a Gilead Company, Merck, Bristol Myers Squibb, Novartis, Celgene, Pfizer, Allogene, Kuur, Incyte, Precision BioSciences, Legend, Adicet Bio, Calibr, and Unum Therapeutics: Other: personal fees; Kite, a Gilead Company, Bristol Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics (Cogent Biosciences), Allogene, Precision BioSciences, Acerta and Adicet Bio: Research Funding; Takeda Pharmaceuticals and related to cell therapy: Patents & Royalties. Nastoupil: MorphoSys: Honoraria; Bayer: Honoraria; IGM Biosciences: Research Funding; Janssen: Honoraria, Research Funding; ADC Therapeutics: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Gilead/Kite: Honoraria, Research Funding; Takeda: Honoraria, Other: DSMC, Research Funding; Denovo Pharma: Other: DSMC; TG Therapeutics: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Caribou Biosciences: Research Funding; Epizyme: Honoraria, Research Funding. Ahmed: Seagen: Research Funding; Merck: Research Funding; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Research Funding.
Clinical trial evaluating a novel usage of FDA approved therapies

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal